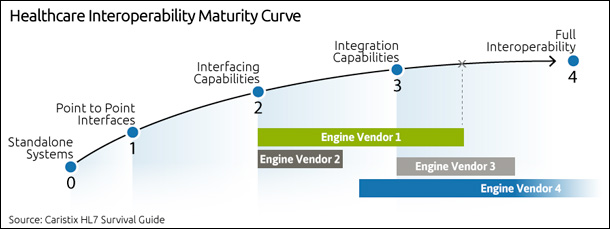
When you first start researching the interfacing capabilities of each engine vendor, you might be overwhelmed. If you’re in a startup, small company, or a hospital with fewer than 100 beds, get ready for sticker shock. Interfacing and integration don’t come cheap.
And if you’re in a bigger organization and you need a new engine, get ready for complexity and value propositions that may be overkill.
This post will help you match your needs to the capabilities in the market. And we’ll look at the hidden costs beyond the engine technology.
Point to point
If you’re just getting started with interfacing, you’ll want to consider simple point-to-point connections. These are interfaces that are custom-built between two systems, and they don’t go through an engine. The advantage? You can get up and running quickly. But this technique is hard to scale beyond a handful of systems, since every connection requires its own interface. Also, watch for HIT vendor costs. One thing that suprises teams that are new to interfacing is that many EMR and HIT vendors charge for access. Even if you build your own interface, they will charge a licensing fee for 3rd party access to the data in their systems. And if you rely on these vendors to change or add new interfaces, the cost rises quickly.
Interfacing capabilities
Once your needs expand beyond a handful of interfaces, you’ll look for an interface engine. While the complexity of coding varies from one engine to another, expect a learning curve and expect to invest time to get up to speed.
On the other hand, you’ll find that engines make it easier to handle changes from one transport protocol to another – for instance, blending TCP/IP and web services. And you’ll find that per-interface costs can drop, since you’ll reduce your dependency on EMR and HIT vendors for changes to their message formats.
Integration, workflow management, and analytics
To meet the growing needs of IDNs and HIEs, many engine vendors now offer greater integration and workflow management capabilities. So for instance, you’ll find workflow management features that allow an interface to change as clinical workflows shift.
Another capability? Data analytics. Over the past 18 to 24 months, some vendors have been promoting their ability to work directly with the data flowing through the engine. You can query clinical and operational data directly to improve processes, decision-making, and care. This is powerful. But where you may run into issues is in the infrastructure. Just be aware that your infrastructure may need expensive upgrades in order to use these capabilities.
The real costs
Now that we’ve looked at the capabilities, let’s turn to the costs. Sure, you’ll invest in licensing and infrastructure – software and hardware. But your biggest line item will be the development costs.
What are the hidden development costs?
First, some interfaces are just more complex than others. For instance, a lab order interface may take twice as long to create as a registration or scheduling interface. But overall, interface development costs fit a pattern, which breaks down as follows.
Research and specification development
- Reviewing product specs, developing a documentation template for project communications and populating it.
Gap analysis, mapping tables
- Reviewing codes in both systems. Understanding differences in code sets. Creating mapping tables.
Interface build or coding
- Actually writing the interface in the engine or coding by hand.
Unit testing
- Testing each piece as you build the interface. This iterative process can take 10% of your coding time.
Integration testing
- Testing clinical or operational workflows that the interface enables, in the test environment, before go-live.
Script development
- Are there scripts that have to be developed for your interface to work? Expect development time and costs if this is the case.
Documentation
- You want this handed off right so the interface can be maintained. Read more about documentation in this post.
Communications costs
- A hidden cost that can escalate, especially if you don’t have a collaboration platform to keep track of versioning.
Post go-live support
- The more applications you have, the higher the costs will be.
Reducing development costs
You can reduce your development costs by incorporating interface lifecycle management into your technology stack. Interface lifecycle management software can reduce the time and cost of research and specification development, gap analysis, testing, and more. As your requirements become more complex, the more you’ll bring your costs down with interface lifecycle management best practices and software.
Check out Caristix Workgroup for a full interface lifecycle management suite (16-minute on-demand demo available).