First, what’s the problem?
First, what’s the problem?
One of the problems we hear about from HL7 team leads and their management is that interfaces take a long time to configure. The reasons why boil down to missing information. Here’s what they typically hear back from the team members:
“The fields need fine-tuning.”
“We don’t know up-front which message types will need the most work.”
“That field mapping was a bear because we couldn’t get a complete code set from the source and destination systems.”
“These code sets have been customized. Each code set changes with the message type.”
Impact: money and time
Integration team leads worry when they hear these statements because these bottlenecks signal delays and project slippage. Problems with configuration prolong testing cycles. They push out go-live – or push burnt-out team members to work even longer hours. It’s demoralizing for the analyst and developer, and frustrating for clinical testers. And the bottom line is: it’s a huge business concern for leadership, who see money and time going down the drain with delays.
There is a way out. It’s called gap analysis.
Just what is gap analysis? Gap analysis is about getting the mapping information upfront. In other words, you compare specs for both the source and destination systems upfront, before a single line of interface code is written and or dragged-and-dropped in the engine.
Your gap analysis must answer the following basic questions:
- Do the source and destination systems handle the same message types? If not, what are the differences?
- Are there mandatory elements in one system that are optional in the other? Note them down.
- Do both systems use the same code sets? With the same values? If not, which values need to be mapped? Do this for every field that needs to be mapped.
- Do both systems require the same maximum length for all fields? Note the exceptions.
- Does either system use z-segments? How will you handle mapping?
Document your gap analysis
When you consider the number of message types, segments, and fields that are typically needed in an interface, you won’t want to leave gathering this knowledge to a simple scratch pad. You need a template or system to get everything right and hand off this knowledge to a developer.
Some analysts develop Excel templates to manage all this information. We’ve seen templates with 12 to 15 tabs to track. Excel templates also need to be filled out manually. An analyst spends days comparing two product specs side by side and checking sample messages. Then recording their findings across the Excel tabs.
The other option is to look for interface lifecycle management software that will automate the gap analysis process for you. You want software that will compare two system specs side by and side and flag only the differences that will impact the interface.
And the real reason to automate gap analysis: you’ll save even more time. And time = money.
Further resources
Check out Caristix Workgroup for a full interface lifecycle management suite (16-minute on-demand demo available).
Image credit: https://flic.kr/p/9BsXFM



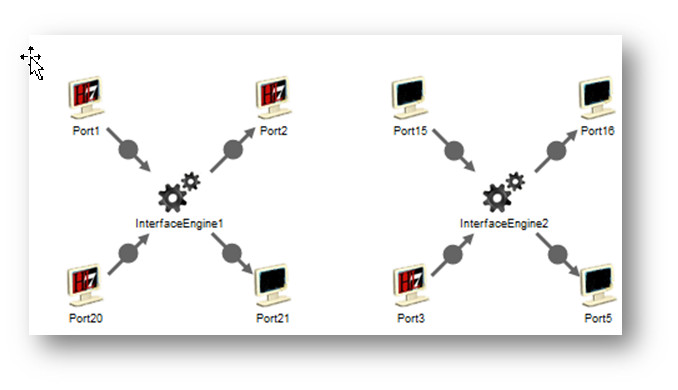

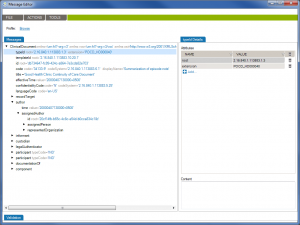
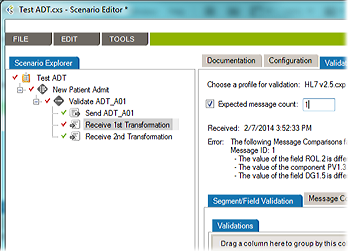
 In last week’s tip on
In last week’s tip on  Over the past few weeks, we’ve reviewed multiple HL7 testing topics. So you know how critical it is to test interfaces during interface configuration, the validation phase, and during maintenance. This HL7 interface testing checklist will help you design a testing process that covers your most important needs. And if you already have a testing process in place, it will help you identify any areas of concern.
Over the past few weeks, we’ve reviewed multiple HL7 testing topics. So you know how critical it is to test interfaces during interface configuration, the validation phase, and during maintenance. This HL7 interface testing checklist will help you design a testing process that covers your most important needs. And if you already have a testing process in place, it will help you identify any areas of concern.